Outcomes for patients with acute lymphoblastic leukemia (ALL) continue to improve in the era of immune- therapies. Despite this, measurable residual disease (MRD) frequently persists after frontline treatment, necessitating allogeneic hematopoietic stem cell transplantation or prolonged chemotherapy lasting multiple years. Relapse remains common and is associated with long-term survival rates of <25%. This emphasizes the need for research into fundamental mechanisms that enable the persistence of leukemic blasts.
We previously showed that adding anti-PDL1 therapy to the tyrosine kinase inhibitor nilotinib improved survival from 10% to 70% in a murine model of BCR-ABL+ ALL. This effect was abrogated in the absence of CD4 T cells, suggesting a key role for CD4 T cells in anti-leukemia immunity. Persisting MRD in the absence of PDL1 blockade was associated with the polyclonal expansion of FOXP3- CD4 T-cells. These CD4s expressed high levels of the suppressive cytokine IL10, and loss of key helper molecules such as CD40L. In contrast, successful treatment with PDL1 blockade correlated with oligoclonal expansion of a CD4+ T-cell subset with preserved CD40L expression and diminished IL10.
The observed IL10+ FOXP3- CD4 T-cell phenotype implies a T-regulatory type 1 (Tr1) identity. Importantly, a specialized Tr1 subset has been shown to eliminate mutation-harboring hematopoietic stem cells (HSCs), demonstrating a role for surveillance and leukemia prevention by Tr1s in the HSC niche. Tr1s convey pro-proliferative and differentiation signals towards mutation-harboring HSCs, driving their development into short-lived myeloid blasts. Tr1s in the HSC niche secrete high levels of IL10, apparently to minimize excess inflammation while eliminating mutated HSCs. In our current proposal, we hypothesize that the pro-proliferative and immunosuppressive signals of Tr1s are co-opted by residual acute lymphoblastic leukemia cells, maladaptively protecting them from immune attack and eradication.
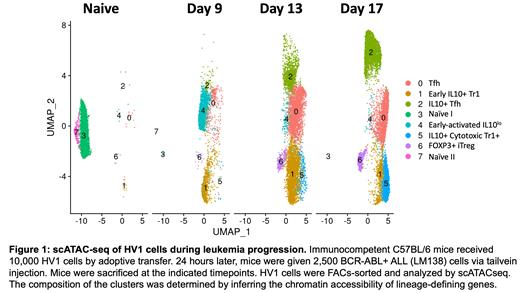
To investigate this hypothesis, we made a novel transgenic mouse (HV1) that harbors CD4 T-cells with a fixed TCR derived from the oligoclonally expanded CD4+ T-cells that we observed during treatment with anti-PDL1 blockade. HV1 CD4 T-cells specifically recognized and expanded in response to BCR-ABL+ ALL cells. HV1 cells remained largely FOXP3-negative and demonstrated an increase in IL10 expression with concordant loss of CD40L during leukemia progression. scATACSeq studies revealed that naïve HV1 cells develop into 5 distinct subsets during leukemia challenge in vivo (Fig. 1). Accessible chromatin regions (ACRs) of IL10, EOMES and other Tr1-like genes were high in clusters 1,2, and 5. Interestingly, clusters 1 and 5 did not have a loss of ACRs in genes associated with helper/effector function (CD40L, IFNγ, TNF). Functional studies verified that co-culture of CD4 T-cells with BCR-ABL+ ALL cells induced CD4s to suppress bystander CD8+ T-cells. Adoptive transfer of HV1 cells prolonged survival of mice bearing BCR-ABL+ ALL, but was non-curative, consistent with reprogramming of HV1 cells to an IL10+ suppressive phenotype.
These findings imply that anti-leukemia CD4 T-cells in the presence of leukemia initially develop into heterogenous pro-helper/cytotoxic subsets. Chronic exposure to leukemia drives most of these early subsets to terminally differentiate into IL10+/FOXP3- suppressive Tr1s. These Tr1s do not bear classic hallmarks of exhaustion (e.g. epigenetic silencing of effector cytokines) but instead phenocopy a class of CD4s that normally guards the HSC niche. Anti-PDL1 blockade may function in this context by clonally expanding progenitor helper/cytotoxic leukemia-specific CD4s, tilting the local balance away from IL10+ populations. This mechanism is distinct from rescue of early-exhausted T-cell populations, which is a well-accepted explanation for the effects of checkpoint blockade on CD8+ T-cells in solid tumor settings. Our results imply a novel mechanism underlying anti-PDL1 checkpoint blockade clinical efficacy, which may be therapeutically exploited to eradicate measurable residual disease.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal